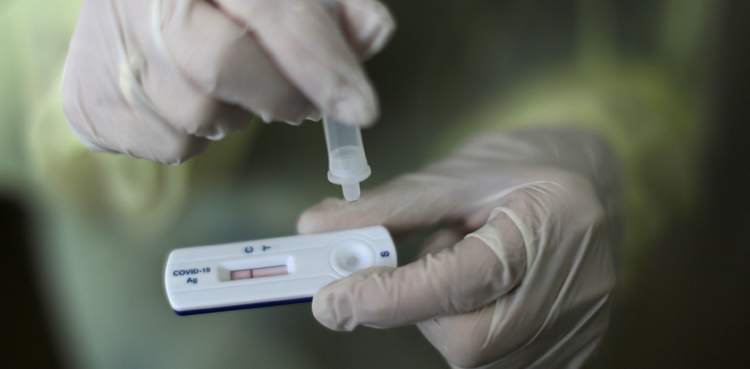
ISLAMABAD: The Drug Regulatory Authority of Pakistan (DRAP) has given the approval to the COVID-19 rapid testing kit, citing sources.
The chief executive officer (CEO) of DRAP, Dr Asim Rauf, confirmed that a foreign pharmaceutical company has registered the coronavirus rapid testing kit which will provide the result of a nasal swab sample within 20 minutes.
Dr Rauf said the testing kits manufactured in Germany will be imported by a foreign pharmaceutical company and the initial testing fees will be charged up to Rs2,000. Only trained experts will be able to use the rapid testing kits and the results will be matched with the health condition and clinical history of patients, he added.
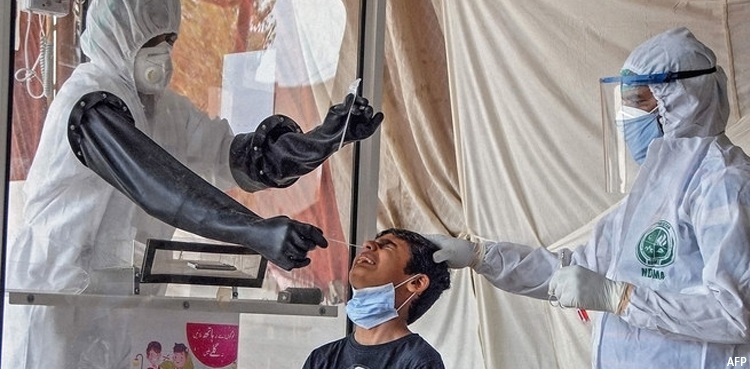
The DRAP CEO said that rapid testing kits will not be used for donor screening and termed the registration of the kits as a major success for speedy diagnosis of the virus. He said that timely diagnosis of the virus will enable the medical experts to treat the infected patients in the country.
Earlier on Saturday, DRAP CEO Dr Asim Rauf had announced Pakistan has locally developed a modern device to diagnose coronavirus within a minute.
The invention is being regarded as a major breakthrough for Pakistan in its fight against the pandemic and it had been approved by the DRAP.
As per details, the device is capable of diagnosing the deadly disease within a minute through the lungs. The device has been named COVID Rapid Artificial Intelligence Detection.
Dr Asim had said that the device has been purely developed at the domestic level and has been approved under the DRAP Act 2012. He had said that the device will be helpful for Pakistan in the war against COVID-19 as it can detect the infection within a minute.
CEO DRAP had said that the device that was developed by National Electronics Complex Pakistan, will be available in the market soon.